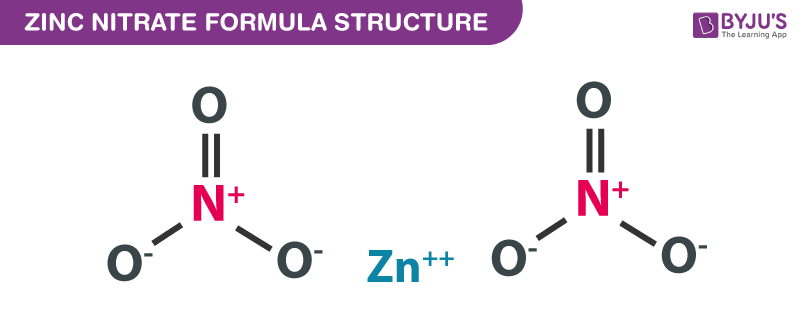
Zinc Nitrate formula, also known as Zinc dinitrate formula or Celloxan formula is explained in this article. This inorganic compound consists of two nitrogen atoms, six oxygen atoms, and a zinc atom. The chemical or molecular formula of Zinc Nitrate is Zn(NO3)2.
It often appears as a colourless to withe crystalline solid. It is highly deliquescent and does dissolve in water and alcohol. It can be synthesized by mixing zinc in nitric acid. It is naturally found as hexahydrate. It is widely used as a catalyst in the manufacturing of chemicals, dyes, and medicines.
Zinc Nitrate Formula Structure
Properties Of Zinc Nitrate Formula
| Chemical formula | Zn(NO3)2 |
| Molecular weight | 189.36 g/mol (anhydrous)
297.49 g/mol (hexahydrate) |
| Density | 2.065 g/cm3 (hexahydrate) |
| Boiling point | Approx 125 °C (hexahydrate) |
| Melting point | 110 °C (anhydrous)
45.5 °C (trihydrate) 36.4 °C (hexahydrate) |
Zn(NO3)2 is a non-combustible compound but it has the ability to enhance the burning of combustible compounds. Inhaling this inorganic chemical may lead to sore throat and severe coughing. When it comes in contact with skin and eyes it causes redness and pain. Ingesting this compound leads to nausea, abdominal pain, etc.
To learn more about Zinc Nitrate formula from the expert faculties at BYJU’S, register now!
Comments